Boycott Teva, boycott Ajovy. Boicot Teva, boicot Ajovy ENG ESP
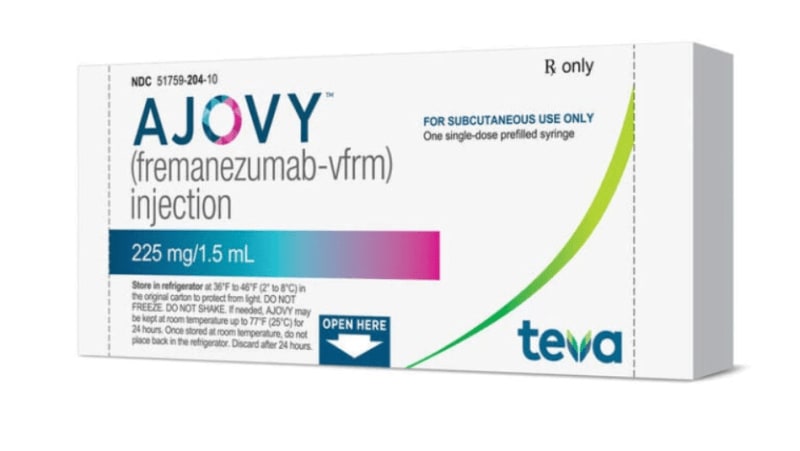
A medication that offers nothing new to "prevent" migraine attacks). Un medicamento que no aporta nada bueno nuevo para “prevenir” crisis de migraña
ENGLISH
Boycott Teva, boycott Ajovy (a medication that offers nothing new to "prevent" migraine attacks)
Health Workers for Palestine, Spain HW4PS
https://hw4p.es/ hcw4palestinespain@gmail.com
Why boycott Teva and Ajovy?
Teva is an Israeli pharmaceutical company that earned $16.4 billion in 2024. It is a corrupt and corrupting company, with billions of dollars in convictions and fines for various criminal acts, in some cases even resulting in deaths (for example, with the overuse of opiates in the United States), 1.
Teva serves as a source of income for Israel, which thus allows it to commit and sustain apartheid and genocide in Palestine, 2.
In Spain, Teva's three best-selling drugs in primary care are omeprazole, atorvastatin, and pantoprazole, which is why Health Workers for Palestine, Spain (HW4PS) is promoting a boycott and their replacement with equivalent drugs, 3.
In hospitals, the best-selling drug is Ajovy, prescribed by hospital specialists and dispensed by hospital pharmacies. Hence this specific boycott proposal.
Ajovy offers nothing new
The French journal Prescrire is probably the best pharmacotherapeutic bulletin in the world. In 2020, its assessment of fremanezumab (Ajovy) concluded with "It offers nothing new"iv (literally, "N'apporte rien de nouveau") in the "prevention" of migraine, 4.
It should be corrected to say “It offers nothing new and beneficial” since it has the adverse effects of other drugs in its class, ranging from constipation and muscle pain to Raynaud's syndrome (adverse effects that are only partially known given its recent introduction to the market), 5, 6.
Ajovy, mechanism of action
Ajovy is a monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) receptor, blocking the action of this neuropeptide,7,8. CGRP is located throughout the nervous system, but is concentrated in the perivascular sensory afferents of the trigeminal nerve and its caudal nucleus. CGRP, which has a vasodilatory effect, appears to be involved in the onset of migraine attacks. Gepants, CGRP inhibitor drugs, are used in the “prevention” of migraine attacks. Ajovy is produced in Chinese hamster ovary cells using recombinant DNA technology,9.
Anti-CGRPs
Among the anti-CGRPs, erenumab (Aimovig° - Novartis Pharma), fremanezumab (Ajovy° - Teva Santé), and galcanezumab (Emgality° - Lilly) are administered subcutaneously; eptinezumab (Vyepti° - Lundbeck) is administered intravenously; and rimegepant (Vydura° - Pfizer) and atogepant (Aquipta – AbbVie) are both administered orally. Rimegepant is the only anti-CGRP also authorized for the treatment of migraine attacks.
All of them present problems of excessive price not adjusted to effectiveness,10.
Inhibition of the vasodilatory action of CGRP creates uncertainty regarding the safety of these drugs in the cardiovascular system of patients with present or latent cardiovascular disease, due to the potential worsening of ischemic events such as stroke, transient ischemic attack, or myocardial infarction, and the possible risk of eclampsia during pregnancy.
Use in practice
The French National Authority for Health (HAS), in 2025, clarified that: “To date, the superiority of anti-CGRP antibodies (including fremanezumab) in terms of efficacy compared to other prophylactic treatments has not been demonstrated.”, 11.
There are no differences in efficacy in the use of eptinezumab, galcanezumab, fremanezumab, and erenumab in chronic migraine, 12. However, it appears that eptinezumab (Vyepti) may reduce migraine days more than fremanezumab (Ajovy) due to its better affinity and specificity when binding to the calcitonin gene-related peptide receptor (CGRP), 13.
As summarized in the Navarre Pharmacotherapeutic Information Bulletin, similarly to the UK's NICE guidelines and the Spanish Therapeutic Positioning guidelines 14,15,16:
“Anti-CGRP monoclonal antibodies represent a new treatment alternative for patients with eight or more migraine days per month who have experienced three or more treatment failures over at least three months, including the administration of botulinum toxin A in the case of chronic migraine. These antibodies show modest efficacy compared to placebo, and there are no comparative studies against standard therapies. Caution should also be exercised in patients with a history of cardiovascular disease.”
The Bulletin highlighted the therapeutic effect of the placebo (almost 40%).
Ajovy is administered subcutaneously, in 225 mg doses, as an injectable solution in a pre-filled syringe, at a price of €498 in the Spanish National Health System (a price that can be reduced by more than 50% in specific offers, as Teva negotiates with this product to gain a strong market position). In the United States, the price is $746.
Boycott Ajovy
Teva presented its shareholder report in November 2025, and regarding Ajovy, it reiterated its projected earnings of around $640 million in 2025 (17).
Ajovy offers nothing new and, in any case, can be replaced by other anti-CGRP drugs.
If you are a patient, refuse this treatment; do not contribute to the suffering of the Palestinian people.
If you are a doctor, choose an alternative that does not support apartheid and genocide in Palestine by Israel.
Patricia Pozo-Reich
In the world of headaches, Patricia Pozo-Rosich holds a key position.
A key opinion leader (KOL, a person elevated by industries to influence their colleagues) and neurologist at Vall d’Hebron University Hospital in Barcelona, a public hospital, she is the head of the Headache Unit at Vall d’Hebron University Hospital in Barcelona. She directs the Migraine Adaptive Brain Center and the Headache and Neurological Pain Research Group - Laboratory at the VHIR (Vall d’Hebron Research Institute), which belongs to the Autonomous University of Barcelona, 18.
She also sees patients at the private QuirónSalud Hospital in Barcelona and is a member of the Scientific Council of the Lilly Foundation.
Patricia Pozo-Rosich received almost €32,000 from Teva in 2023 and €45,000 in 2024, in “transfers of value.”
In 2023, the SEN Foundation (Spanish Society of Neurology), in Barcelona, received €149,645 and the Vall d'Hebron University Hospital Foundation, Research Institute, Barcelona, received €112,850, (19).
The conflicts of interest declared by Patricia Pozo-Rosich are, for example, in the “Guidelines of the International Headache Society for Real-World Evidence studies in migraine and cluster headache”, 20:
“In the last 36 months, she received fees as a consultant and speaker for: AbbVie, Amgen, Dr. Reddy's, Eli Lilly, Lundbeck, Medscape, Novartis, Organon, Pfizer, and Teva. Her research group has received research grants from Novartis, Teva, AbbVie, EraNET Neuron, RIS3CAT FEDER, AGAUR, and ISCIII; and funding for clinical trials from Amgen, Biohaven, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva.”
In 2024, for example, she participated in the publicity-labeled presentation of a gepant, an anti-CGRP, atogepant (Aquipta – AbbVie).
Logically, 21, such conflicts of interest invalidate all consensus statements, clinical trials, guidelines, and reviews on headaches in which Patricia Pozo-Reich has participated, is participating, or will participate.
NOTE:
In Spain, as we mentioned, Patricia Pozo Rosich, a neurologist at Vall d'Hebron Hospital in Barcelona, stands out. She received €77,000 directly from Teva in what they call "transfers of value," totaling €77,000 in 2023 and 2024. The Vall d'Hebron University Hospital Foundation, Research Institute, also in Barcelona, received €138,000. It should be noted that the Vall d'Hebron University Hospital Foundation (VHIR) has the aforementioned neurologist Patricia Pozo Rosich as vice president of its Internal Scientific Committee and Ronit Satchi-Fainaro, an Israeli from Tel Aviv, on its External Scientific Committee. Satchi-Fainaro is a member of Teva's Board of Directors (a fact omitted from her curriculum vitae on the VHIR website).
-Scientific Internal Committee VHIR
https://vhir.vallhebron.com/es/instituto/organos-de-gobierno/comite-cientifico-interno
-Scientific External Committee VHIR
https://vhir.vallhebron.com/es/instituto/organos-de-gobierno/comite-cientifico-externo
Board of Directors: Prof. Ronit Satchi-Fainaro Independent Director
https://www.tevapharm.com/our-company/leadership-governance/board-of-directors/prof-ronit-satchi-fainaro/
Much of the scientific literature on Ajovy is authored by individuals who declare conflicts of interest regarding Teva, or are directly employed by Teva (or Otsuka, the company that markets the drug in Japan), and in many cases, this literature is written by third parties and directly funded by Teva.
Some examples:
- US Real-World Effectiveness, Tolerability, and Healthcare Resource Utilization After Addition of Fremanezumab for Preventive Treatment in Patients Using Gepants for Acute Treatment of Migraine: Results From a Retrospective Chart Review.
https://link.springer.com/article/10.1007/s12325-024-03063-w#Fun
- This study, the journal's rapid service, and the open access fee were funded by Teva Pharmaceuticals.
-Patrick Eugeni, Megan E. Rooney, Nicolas P. Saikali, Zhongzheng Niu, and Jennifer W. McVige are employees of the Dent Neurological Institute, which conducted this study funded by Teva Pharmaceuticals. Jennifer W. McVige has received research funding from AbbVie, Teva Pharmaceuticals, Eli Lilly, Amgen, and Lundbeck; and lecture and consulting fees from AbbVie, Teva Pharmaceuticals, Eli Lilly, Amgen, Lundbeck, Avanir, Theranica, and Amneal. Maurice T. Driessen and Lynda J. Krasenbaum are employees of Teva Pharmaceuticals. Karen Carr and Michael J. Seminerio are former employees of Teva Pharmaceuticals (current affiliation: AbbVie, North Chicago, IL, USA).
-Cost Effectiveness of Fremanezumab in Episodic and Chronic Migraine Patients from a Japanese Healthcare Perspective. https://link.springer.com/article/10.1007/s40273-024-01380-0
-This work was funded by Otsuka Pharmaceuticals Co., Ltd. The funding entity oversaw the conduct of the study and the preparation of the manuscript. Otsuka Pharmaceuticals Co., Ltd. paid the open access fee.
-Takao Takeshima declares having received consulting fees from Otsuka, Amgen, Eli Lilly, Daiichi Sankyo, Shionogi, Bioheaven, and Lundbeck, and being an advisor to Hedgehog MedTech and Sawai. Fumihiko Sakai declares having received consulting fees from Otsuka, Amgen, Eli Lilly, and Daiichi Sankyo. Xinyu Wang worked for Otsuka Pharmaceutical Co., Ltd. during the conduct of the study and declares no conflicts of interest. Kentaro Yamato, Yoshitsugu Kojima, and Yilong Zhang work for Otsuka Pharmaceutical Co., Ltd. and declare no conflicts of interest. Otsuka Pharmaceutical Co., Ltd. marketed fremanezumab in Japan. Craig Bennison and Martijn Simons work for OPEN Health and report having received consulting fees from Otsuka. OPEN Health is a consulting firm contracted by Otsuka to conduct the study and draft the manuscript.
-Real-world effectiveness of fremanezumab for the preventive treatment of migraine: Interim analysis of the pan-European, prospective, observational, phase 4 PEARL study.
https://pubmed.ncbi.nlm.nih.gov/37987641/
-Medical writing and editorial support for the development of this manuscript, under the authors' direction, were provided by Molly Clark, Sophie Roberts, and Olivia Morris of Ashfield MedComms, an Inizio company, and funded by Teva Pharmaceuticals.
-The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MA declares having received personal fees and having been a principal investigator in clinical trials for AbbVie, Amgen, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva Pharmaceuticals; and research grants from the Lundbeck Foundation, the Novo Nordisk Foundation, and Novartis. DDM has received honoraria, research grants, and travel expenses from Allergan/AbbVie, Amgen, Biogen, Cefaly, Genesis Pharma, Electrocore, Eli Lilly, Lundbeck, Merck-Serono, Mertz, Novartis, Roche, Sanofi, Specifar, and Teva Pharmaceuticals. He served as a principal investigator in clinical trials for Amgen, Cefaly, Electrocore, Eli Lilly, Genesis Pharma, Lundbeck, Mertz, Novartis, Specifar, and Teva Pharmaceuticals; and he is chairman of the board of the Hellenic Headache Society and co-chair of the steering group of the European Academy of Neurology's Headache Panel. FMA reports having lectured, served on advisory boards, and/or served as a principal investigator in clinical trials for Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva Pharmaceuticals. PK, VRC, and AHA are employees and/or shareholders of Teva Pharmaceuticals. LL is a former employee of Teva Pharmaceuticals. CJS reports having received consulting services, participation on advisory boards, presentations, and travel funding from Novartis, Eli Lilly, Teva Pharmaceuticals, AbbVie, Allergan, Almirall, Amgen, Lundbeck, MindMed, and Grünenthal; and is a part-time employee of Zynnon. GS reports having received research funding from the Swiss Heart Foundation, the Eye on Vision Foundation, the Baasch-Medicus Foundation, and the German Migraine and Headache Society. GS reports having participated on advisory committees and/or been a principal investigator in clinical trials for AbbVie, Lundbeck, Novartis, Pfizer, and Teva Pharmaceuticals; and having received research funding from Vinnova (the Swedish innovation agency), Lund University, and the Swedish Neurological Association. PPR declares having received research grants/funding from AbbVie, AGAUR, EraNet NEURON, the Carlos III Health Institute, MINECO, Novartis, RIS3CAT FEDER, and Teva Pharmaceuticals; and having carried out consultancies or training activities for AbbVie, Amgen, Biohaven, Chiesi, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva Pharmaceuticals. PJD declares having participated in training meetings and/or collaborated on clinical trials for Allergan/AbbVie, electroCore, Novartis, Teva Pharmaceuticals, and Lundbeck. TN declares having received fees for consultancy, speaking engagements, and travel grants from Eli Lilly, Glenmark, Lundbeck, Novartis, Pfizer, St. Jude Medical, Teva Pharmaceuticals, and UCB. and have participated on advisory committees or as principal investigator in clinical trials for AbbVie, Amgen, Eli Lilly, Lundbeck, Neurocrine, Novartis, Teva Pharmaceuticals, and UCB. ACP reports having lectured, participated on advisory committees, or served as principal investigator in clinical trials for Allergan, AbbVie, Pfizer, Teva Pharmaceuticals, Novartis, Lundbeck, Eli Lilly, and Roche. IPM reports having lectured, participated on advisory committees, or served as principal investigator in clinical trials for Allergan-AbbVie, Teva Pharmaceuticals, Novartis, Lundbeck, and Eli Lilly. MLS reports having lectured, participated on advisory committees, or served as principal investigator in clinical trials for Pfizer, Teva Pharmaceuticals, Novartis, Lundbeck, AbbVie, and Eli Lilly. CT reports personal fees for participation in advisory boards or symposia for AbbVie, Dompé, Eli Lilly, Lundbeck, Novartis, Pfizer and Teva Pharmaceuticals; reports institutional fees for conducting clinical trials for AbbVie, Dompé, Eli Lilly, Lundbeck, Novartis, Pfizer and Teva Pharmaceuticals; received funding for research projects from the European Commission, the Italian Ministry of Health, the Migraine Research Foundation and the Italian Multiple Sclerosis Foundation.
-A Dutch cost-effectiveness analysis of fremanezumab versus best supportive care in patients with chronic migraine and inadequate response to prior preventive therapy. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-024-03697-x ---This work was supported by Teva Pharmaceuticals.
-SW is an employee of Asc Academics and a consultant to the MH Pronk Health Care Consultancy Foundation. -JAC has received remuneration for serving on advisory boards and for speaking engagements with Teva Pharmaceuticals, Lilly, Novartis, Lundbeck, and Pfizer. MP is the managing director of the MH Pronk Health Care Consultancy Foundation and an external consultant to Teva Netherlands B.V. KWMZ is an employee of Teva Netherlands B.V. MTD is an employee of Teva Pharmaceuticals. LL is a former employee of Teva Pharmaceuticals. MJP is a consultant to Asc Academics.
-Guidelines of the International Headache Society for Real-World Evidence studies in migraine and cluster headache. https://journals.sagepub.com/doi/10.1177/03331024251318016
-The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.P.R. reports having received consulting and speaking fees in the last 36 months from: AbbVie, Amgen, Dr. Reddy's, Eli Lilly, Lundbeck, Medscape, Novartis, Organon, Pfizer, and Teva. Her research group has received research grants from Novartis, Teva, AbbVie, EraNET Neuron, RIS3CAT FEDER, AGAUR, and ISCIII; and clinical trial funding from Amgen, Biohaven, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva. She is Honorary Secretary of the International Headache Society. She is a member of the Clinical Trials Guidelines Committee of the International Headache Society. She is an associate editor of Cephalalgia, Headache, The Journal of Headache and Pain, Neurologia, and Revista de Neurologia. She is the founder of www.midolordecabeza.org. P.P.R. does not own shares in any pharmaceutical company. N.v.V. has no conflicts of interest. E.C. has received fees from Novartis, Chiesi, Lundbeck, and MedScape; her salary has been partially funded by the Rio Hortega Strategic Action in Health 2017–2020 and Juan Rodés 2024–2028 grants from the Carlos III Health Institute (CM20/00217). She is a junior editor of Cephalalgia. G.V. has no conflicts of interest. M.T.F. has received fees from AbbVie, Novartis, Chiesi, and Teva. B.W.H.v.d.A. P.J.G. reports having received independent support from the Netherlands Research Council (849200007) and the Netherlands Brain Foundation (HA2017.01.05). P.J.G. also reports having received, during the last 36 months, a grant from Celgene; personal fees from Aeon Biopharma, Abbvie, Amgen, CoolTech LLC, Dr. Reddy's, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, Shiratronics, Teva Pharmaceuticals, and Tremeau; personal fees for consulting through Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and royalties or fees for publications from the Massachusetts Medical Society, Oxford University Press, UpToDate, and Wolters Kluwer. and a patent for magnetic stimulation for headache (no. WO2016090333 A1) assigned to eNeura free of charge. M.A. declares having received fees from AbbVie, Amgen, Eli Lilly, GlaxoSmithKline, Lundbeck, Novartis, Pfizer, and Teva Pharmaceuticals, outside the scope of the submitted work. M.A. is also an associate editor of Brain, Cephalalgia, and The Journal of Headache and Pain. S.J.W. has served on the advisory boards of Allergan and Eli Lilly Taiwan. He has received speaking fees from the Taiwanese subsidiaries of Pfizer, Eli Lilly, and GSK, as well as research grants from the National Science and Technology Council of Taiwan, Taipei Veterans General Hospital, and the Taiwan Headache Society. H.C.D. is a member of the Clinical Guidelines Committee of the German Society of Neurology and the Clinical Trials Committee of the IHS. C.T. has received fees for participation on advisory boards and/or at scientific symposia for AbbVie, Dompé, Eli Lilly, Medscape, Novartis, Lundbeck, and Teva. His institution has received payments from AbbVie, Eli Lilly, Novartis, Lundbeck, and Teva for investigator-sponsored or clinical trials. G.M.T. reports receiving consulting or industry support from AbbVie, Lilly, Lundbeck, Novartis, Pfizer, Teva, and Interactive Studios BV, as well as independent support from the European Community, the Dutch Heart and Brain Foundation, the Netherlands Research Council, and Dioraphte. -Calcitonin Gene-Related Peptide Monoclonal Antibodies: Key Lessons from Real-World Evidence. https://www.mdpi.com/2076-3425/14/9/948 -Piero Barbanti has received remuneration for consulting services, participation on a scientific advisory board, lectures, research support, and participation in clinical trialsor other activities with companies such as Abbvie, Alder, Allergan, Amgen, Angelini, Assosalute, Bayer, Biohaven, Eli Lilly, Fondazione Ricerca e Salute, GSK, Lundbeck, New Penta, Noema Pharma, Novartis, Pfizer, Organon, Teva, Visufarma, and Zambon. She is also president of the Italian Association of Headache Sufferers. Cinzia Aurilia has received travel grants from Eli Lilly, FB-Health, Lusofarmaco, and Teva, as well as fees from Novartis, Eli Lilly, and Teva. Gabriella Egeo has received travel grants and fees from Eli Lilly, Novartis, New Penta, and Ecupharma. Bianca Orlando and Giulia Fiorentini have nothing to declare.
1.-Teva en España. Un gigante farmacéutico israelí cuyas ganancias financian en genocidio en Palestina. https://www.nogracias.org/2025/01/01/teva-en-espana-un-gigante-farmaceutico-cuyas-ganancias-danan-la-salud-de-la-poblacion-de-palestina-profesionales-de-la-sanidad-por-palestina-espana-healthworkers4palestinespain/
2.-The heroes of Teva. https://www.jpost.com/special-content/the-heroes-of-teva-779380
3.-Boicot (acción directa) a Teva. Dirigida contra sus 3 "superventas". 1/ omeprazol, 2/ atorvastatina, 3/ pantoprazol. https://hw4p.es/blog/boicot-accion-directa-a-teva-dirigida-contra-sus-3-superventas-1-omeprazol-2-atorvastatina-3-pantoprazol
4.-Frémanezumab (Ajovy°) et prévention des crises de migraine. Rev Prescrire 2020 ; 40 (444): 731-732. https://www.prescrire.org/avis-sur-les-medicaments/444-fremanezumab-ajovy-et-prevention-des-crises-de-migraine
5.-CGRP Monoclonal Antibodies (MABs): Risks and Side Effects. https://migrainecanada.org/cgrp-monoclonal-antibodies-mabs-risks-and-side-effects/
6.-Disproportionality analysis of Raynaud’s phenomenon associated with calcitonin gene-related peptide inhibitors using the Food and Drug Administration adverse event reporting system. https://www.nature.com/articles/s41598-025-87421-w
7.-CGRP inhibitors. https://www.drugs.com/drug-class/cgrp-inhibitors.html
8.-Calcitonin Gene-Related Peptide Receptor. https://www.ncbi.nlm.nih.gov/sites/books/NBK560648/
9.-Ficha Técnica Ajovy 225 mg solución inyectable en jeringa precargada. https://cima.aemps.es/cima/dochtml/ft/1191358001/FT_1191358001.html
10.-Antimigraineux anti-CGRP : mi-2024, un accès au prix fort en ville. https://www.prescrire.org/strategies-de-soins/487-antimigraineux-anti-cgrp-mi-2024-un-acces-au-prix-fort-en-ville
11.-AJOVY (frémanezumab) - Migraine. https://www.has-sante.fr/jcms/p_3601431/fr/ajovy-fremanezumab-migraine
12.Comparación de la eficacia de eptinezumab, galcanezumab, fremanezumab y erenumab en migraña crónica https://www.ilaphar.org/wp-content/uploads/2024/01/Org_tto_migrana_2311091.pdf
13-A Comparison of Fremanezumab and Eptinezumab in the Prevention of Chronic Episodic Migraine. https://wjbphs.com/content/comparison-fremanezumab-and-eptinezumab-prevention-chronic-episodic-migraine
14.-Fremanezumab for preventing migraine NICE
https://www.nice.org.uk/guidance/TA764/chapter/1-Recommendations
15.-Informe de Posicionamiento Terapéutico de fremanezumab (Ajovy®) en la profilaxis de migraña https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT_11-2020-fremanezumab-Ajovy.pdf
16.-Qué queda y qué hay de nuevo en el manejo farmacológico de la migraña.
https://www.navarra.es/NR/rdonlyres/9B1E99CB-9C9E-4939-B978-856C45827EAE/491139/Bit_v29n1Mod.pdf
17.-Teva Third quarter 2025 Teva Pharmaceutical Industries Ltd. November 5, 2025 https://s24.q4cdn.com/720828402/files/doc_financials/2025/q3/Q325-Earnings-Presentation.pdf
18.-Patricia Pozo-Rosich. Bio. http://pozorosich.com/bio
19.-Teva en España. Un gigante farmacéutico cuyas ganancias dañan la salud de la población de Palestina. https://www.sinpermiso.info/textos/teva-en-espana-un-gigante-farmaceutico-israeli-cuyas-ganancias-financian-el-genocidio-en-palestina
20.-Guidelines of the International Headache Society for Real-World Evidence studies in migraine and cluster headache. https://journals.sagepub.com/doi/10.1177/03331024251318016
21.-Industrias sanitarias, profesionales y pacientes. Cómo evitar una relación con frecuencia tóxica (corrupta). https://accesojustomedicamento.org/industrias-sanitarias-profesionales-y-pacientes-como-evitar-una-relacion-con-frecuencia-toxica-corrupta/
ESPAÑOL
Profesionales de la Sanidad por Palestina (Health Workers for Palestine, Spain HW4PS)
https://hw4p.es/ hcw4palestinespain@gmail.com
¿Por qué el boicot a Teva y el boicot a Ajovy?
Teva es una industria farmacética israelí que ganó 16.400 millones de dólares en 2024. Es una empresa corrupta y corruptora, con miles de millones de dólares en condenas y multas por actos criminales varios, en algunos casos incluso con muertos (por ejemplo, con el sobreuso de opiáceos en Estados Unidos).
Teva sirve de fuente de ingresos a Israel, que así puede cometer y sostener el apartheid y el genocidio en Palestinai,ii.
En España, los tres superventas de Teva en atención primaria son omeprazol, atorvastatina y pantoprazol, y por ello se promueve por Profesionales de la Sanidad Por Palestina (HW4PS, Health Workers for Palestine, Spain) su boicot y su sustitución por fármacos equivalentesiii.
En hospitales, el superventa es Ajovy, de prescripción por especialistas hospitalarios y dispensación por la farmacia hospitalaria. De ahí esta propuesta concreta de boicot.
Ajovy, no aporta nada bueno nuevo
La revista francesa Prescrire es, probablemente, el mejor boletín farmacoterapéutico del mundo. En 2020, su valoración de fremanezumab (Ajovy) concluyó con “No aporta nada nuevo”iv (en francés, literal, “N'apporte rien de nouveau”) en la “prevención” de la migraña.
Habría que corregir y decir “No aporta nada bueno nuevo” puesto que tiene los efectos adversos de los medicamentos de su grupo, desde estreñimiento y dolores musculares a sindrome de Raynaud (efectos adversos que se conocen sólo en parte dada su reciente introducción en el mercado)v,vi.
Ajovy, mecanismo de acción
Ajovy es un anticuerpo monoclonal que se une al receptor del péptido relacionado con el gen de la calcitonina (CGRP, por su denominación en inglés: “calcitonin gene-related peptide”), bloqueando la acción de este neuropéptidovii,viii. El CGRP está ubicado en todo el sistema nervioso, pero se concentra en las aferentes perivasculares sensoriales del nervio trigémino y su núcleo caudalis. El CGRP, que tiene un efecto vasodilatador, parece estar implicado en el inicio de las crisis de migraña.
Los gepantes, medicamentos inhibidores del CGRP, se emplean en la “prevención” de las crisis de migraña.
Ajovy es producido en células de ovario de hámster chino mediante tecnología de ADN recombinanteix.
Anti-CGRP
Entre los anti-CGRP, erenumab (Aimovig° - Novartis Pharma), fremanezumab (Ajovy° - Teva Santé) y galcanezumab (Emgality° - Lilly) se administran por vía subcutánea; eptinezumab (Vyepti° - Lundbeck) se administra por vía intravenosa; y rimegepant (Vydura° - Pfizer) y atogepant (Aquipta – AbbVie), ambos por vía oral. Rimegepant es el único anti-CGRP autorizado también para el tratamiento de las crisis de migraña.
Todos ellos plantean problemas de excesivo precio no ajustado a la efectividadx.
La inhibición de la acción vasodilatadora del CGRP, crea incertidumbre sobre la seguridad de estos fármacos en el aparato cardiovascular en pacientes con enfermedad cardiovascular presente o latente, por posible empeoramiento de acontecimientos isquémicos como ictus, accidente isquémico transitorio o infarto de miocardio y el posible riesgo de eclampsia en el embarazo.
Utilización en la práctica
La Haute Autorité de Santé (HAS) francesa, en 2025, dejó claro que: “Hasta la fecha, no se ha demostrado la superioridad de los anticuerpos anti-CGRP (incluido el fremanezumab) en términos de eficacia en comparación con otros tratamientos profilácticos”xi.
No hay diferencias de eficacia en el uso de eptinezumab, galcanezumab, fremanezumab y erenumab en migraña crónicaxii. No obstante, parece que eptinezumab (Vyepti) podría reducir más los días de migraña que fremanezumab (Ajovy) por su mejor afinidad y especificidad al ligarse con el receptor del péptido relacionado con el gen de la calcitoninaxiii.
Como resumió el Boletín de Información Farmacoterapéutica de Navarra, en forma parecida al NICE inglésxiv y al Posicionamiento Terapéutico españolxv: “Los anticuerpos monoclonales anti-CGRP suponen una nueva alternativa de tratamiento en pacientes con ocho o más días de migraña al mes y que hayan presentado tres o más fracasos a tratamientos previos durante al menos tres meses, entre los que debe incluirse la administración de toxina botulínica A en el caso de migraña crónica. Estos anticuerpos presentan una eficacia modesta frente a placebo, no existiendo estudios comparativos frente a terapias estándar, debiendo además tener precaución en pacientes con antecedentes cardiovasculares”xvi. Dicho Boletín destacó el efecto terapéutico del placebo (casi del 40%).
Ajovy se administra por vía subcutánea, en dosis de 225 mg, solución inyectable en jeringa precargada, a un precio en el Sistema Nacional de Salud en España de 498 euros (precio que puede rebajarse más del 50% en ofertas concretas, como mercancia con la que negocia Teva para conseguir una buena posición de mercado). En Estados Unidos el precio es de 746 dólares.
Boicot a Ajovy
Teva presentó el informe para accionistas en noviembre de 2025, y sobre Ajovy insistió en su proyección de ganancias en torno a los 640 millones de dólares en 2025xvii.
Ajovy no aporta nada nuevo y, en todo caso, puede ser substituido por otros anti-CGRP.
Si es paciente, rechace tal tratamiento, no contribuya al sufrimiento del pueblo palestino.
Si es médico, seleccione una alternativa que no apoye el apartheid y el genocidio en Palestina por Israel.
Patricia Pozo-Reich
En el “mundo” de las cefaleas y en España es clave la posición de Patricia Pozo-Rosich, “key opinion leader” (KOL, “líder de opinión”, persona que encumbran las industrias para influir en sus colegas) y neuróloga en el Hospital Universitario Vall d’Hebron University de Barcelonaxviii,xix. Es la responsable de la Unidad de Cefalea del Hospital Universitario Vall d’Hebron en Barcelona. Dirige el Migraine Adaptive Brain Center y el Grupo de Investigación - Laboratorio de Investigación en Cefalea y Dolor Neurológico del VHIR (Instituto de Investigación del Vall d’Hebron) que pertenece a la Universitat Autònoma de Barcelona.También pasa consulta en el Hospital QuirónSalud de Barcelonaxx y es vocal del Consejo Científico de la Fundación Lillyxxi.
Patricia Pozo-Rosich recibió de Teva casi 32.000 euros en 2023, y 45.000 en 2024, en “transferencias de valor”.
En 2023, la Fundación SEN (Sociedad Española de Neurología), en Barcelona, recibió 149.645 euros y la Fundació Hospital Universitari Valle D’Hebron, Institut de Recerca, Barcelona, 112.850xxii.
Los conflictos de interés declarados por Patricia Pozo-Rosich son, por ejemplo en las “Guidelines of the International Headache Society for Real-World Evidence studies in migraine and cluster headachexxiii”,:
“En los últimos 36 meses, recibió honorarios como consultora y ponente para: AbbVie, Amgen, Dr. Reddy's, Eli Lilly, Lundbeck, Medscape, Novartis, Organon, Pfizer y Teva. Su grupo de investigación ha recibido subvenciones de investigación de Novartis, Teva, AbbVie, EraNET Neuron, RIS3CAT FEDER, AGAUR e ISCIII; y financiación para ensayos clínicos de Amgen, Biohaven, Eli Lilly, Lundbeck, Novartis, Pfizer y Teva”.
En 2024, por ejemplo, participó en la presentación con publicidad de un gepante, anti-CGRP, el atogepant (Aquipta – AbbVie)xxiv,xxv.
En buena lógicaxxvi, tales conflictos de interés invalidan todas los consensos, ensayos clínicos, guías y revisiones sobre cefaleas en que ha participado, participa o participará Patricia Pozo-Reich.
NOTA
Como hemos comentado, en España destaca, sumando el total en 2023 y 2024, Patricia Pozo Rosich, Barcelona, neuróloga del Valle D’Hebron, que recibió directamente de Teva 77.000 euros en lo que llaman “transferencias de valor”. También la Fundació Hospital Universitari Valle D'Hebron, Institut de Recerca, Barcelona, 138.000.
Hay que destacar que la Fundación Hospital Universitari Vall d´Hebrón (Vall d’Hebron Institut de Recerca, VHIR) tiene en su Comité Científico Interno como vicepresidenta a la citada neuróloga Patricia Pozo Rosich y en su Comité Científico Externo a Ronit Satchi-Fainaro, israelí de Tel Aviv, que pertenece al Consejo de Administración de Teva (lo que se oculta en su curriculum que aparece en la página del VHIR).
-Comité Científico Interno del VHIR
https://vhir.vallhebron.com/es/instituto/organos-de-gobierno/comite-cientifico-interno
-Comité Científico Externo del VHIR
https://vhir.vallhebron.com/es/instituto/organos-de-gobierno/comite-cientifico-externo
Board of Directors: Prof. Ronit Satchi-Fainaro Independent Director
https://www.tevapharm.com/our-company/leadership-governance/board-of-directors/prof-ronit-satchi-fainaro/
Gran parte de la literatura científica sobre Ajovy va firmada por autores que declaran conflictos de interés respecto a Teva, o son directamente empleados de Teva (o de Otsuka, la firma que comercializa el medicamento en Japón) y en muchos dicha literatura está escrita por terceros y directamente financiada por Teva.
Algunos ejemplos:
- US Real-World Effectiveness, Tolerability, and Healthcare Resource Utilization After Addition of Fremanezumab for Preventive Treatment in Patients Using Gepants for Acute Treatment of Migraine: Results From a Retrospective Chart Review.
https://link.springer.com/article/10.1007/s12325-024-03063-w#Fun
-Este estudio, el servicio rápido de la revista y la tarifa de acceso abierto fueron financiados por Teva Pharmaceuticals.
-Patrick Eugeni, Megan E. Rooney, Nicolas P. Saikali, Zhongzheng Niu y Jennifer W. McVige son empleados del Dent Neuroological Institute, que realizó este estudio financiado por Teva Pharmaceuticals. Jennifer W. McVige ha recibido financiación para investigación de AbbVie, Teva Pharmaceuticals, Eli Lilly, Amgen y Lundbeck; y honorarios por conferencias y consultoría de AbbVie, Teva Pharmaceuticals, Eli Lilly, Amgen, Lundbeck, Avanir, Theranica y Amneal. Maurice T. Driessen y Lynda J. Krasenbaum son empleados de Teva Pharmaceuticals. Karen Carr y Michael J. Seminerio son ex empleados de Teva Pharmaceuticals (afiliación actual: AbbVie, North Chicago, IL, EE. UU.).
-Cost Effectiveness of Fremanezumab in Episodic and Chronic Migraine Patients from a Japanese Healthcare Perspective. https://link.springer.com/article/10.1007/s40273-024-01380-0
-Este trabajo fue financiado por Otsuka Pharmaceuticals Co., Ltd. La entidad financiadora supervisó la realización del estudio y la preparación del manuscrito. Otsuka Pharmaceuticals Co., Ltd. pagó la tarifa de acceso abierto.
-Takao Takeshima declara haber recibido honorarios por consultoría de Otsuka, Amgen, Eli Lilly, Daiichi Sankyo, Shionogi, Bioheaven y Lundbeck, y ser asesor de Hedgehog MedTech y Sawai. Fumihiko Sakai declara haber recibido honorarios por consultoría de Otsuka, Amgen, Eli Lilly y Daiichi Sankyo. Xinyu Wang trabajaba para Otsuka Pharmaceutical Co., Ltd. durante la realización del estudio y declara no tener conflictos de interés. Kentaro Yamato, Yoshitsugu Kojima e Yilong Zhang trabajan para Otsuka Pharmaceutical Co., Ltd. y declaran no tener conflictos de interés. Otsuka Pharmaceutical Co., Ltd. comercializó fremanezumab en Japón. Craig Bennison y Martijn Simons trabajan para OPEN Health y declaran haber recibido honorarios de consultoría de Otsuka. OPEN Health es una consultora contratada por Otsuka para realizar el estudio y redactar el manuscrito.
-Real-world effectiveness of fremanezumab for the preventive treatment of migraine: Interim analysis of the pan-European, prospective, observational, phase 4 PEARL study.
https://pubmed.ncbi.nlm.nih.gov/37987641/
-La redacción médica y el apoyo editorial para el desarrollo de este manuscrito, bajo la dirección de los autores, fueron proporcionados por Molly Clark, Sophie Roberts y Olivia Morris de Ashfield MedComms, una empresa de Inizio, y financiados por Teva Pharmaceuticals.
-Los autores declaran los siguientes posibles conflictos de interés con respecto a la investigación, la autoría y/o la publicación de este artículo: MA declara haber recibido honorarios personales y haber sido investigador principal en ensayos clínicos para AbbVie, Amgen, Eli Lilly, Lundbeck, Novartis, Pfizer y Teva Pharmaceuticals; y subvenciones para investigación de la Fundación Lundbeck, la Fundación Novo Nordisk y Novartis. DDM ha recibido honorarios, subvenciones para investigación y viajes de Allergan/AbbVie, Amgen, Biogen, Cefaly, Genesis Pharma, Electrocore, Eli Lilly, Lundbeck, Merck-Serono, Mertz, Novartis, Roche, Sanofi, Specifar y Teva Pharmaceuticals. Participó como investigador principal en ensayos clínicos para Amgen, Cefaly, Electrocore, Eli Lilly, Genesis Pharma, Lundbeck, Mertz, Novartis, Specifar y Teva Pharmaceuticals; y es presidente de la junta directiva de la Sociedad Helénica de Cefaleas y copresidente del grupo directivo del Panel de Cefaleas de la Academia Europea de Neurología. FMA declara haber impartido conferencias, participado en consejos asesores y/o haber sido investigador principal en ensayos clínicos para Eli Lilly, Lundbeck, Novartis, Pfizer y Teva Pharmaceuticals. PK, VRC y AHA son empleados y/o accionistas de Teva Pharmaceuticals. LL es un exempleado de Teva Pharmaceuticals. CJS declara haber recibido servicios de consultoría, participación en consejos asesores, presentaciones y financiación para viajes de Novartis, Eli Lilly, Teva Pharmaceuticals, AbbVie, Allergan, Almirall, Amgen, Lundbeck, MindMed y Grünenthal; y es empleado a tiempo parcial de Zynnon. GS declara haber recibido financiación para investigación de la Fundación Suiza del Corazón, la Fundación Eye on Vision, la Fundación Baasch-Medicus y la Sociedad Alemana de Migraña y Cefalea. GS declara haber participado en comités asesores y/o ser investigador principal en ensayos clínicos para AbbVie, Lundbeck, Novartis, Pfizer y Teva Pharmaceuticals; y haber recibido financiación para investigación de Vinnova (la agencia de innovación de Suecia), la Universidad de Lund y la Asociación Neurológica Sueca. PPR declara haber recibido subvenciones/financiación para investigación de AbbVie, AGAUR, EraNet NEURON, el Instituto de Investigación Carlos III, MINECO, Novartis, RIS3CAT FEDER y Teva Pharmaceuticals; y haber realizado consultorías o actividades formativas para AbbVie, Amgen, Biohaven, Chiesi, Eli Lilly, Lundbeck, Novartis, Pfizer y Teva Pharmaceuticals. PJD declara haber participado en reuniones formativas y/o haber colaborado en ensayos clínicos para Allergan/AbbVie, electroCore, Novartis, Teva Pharmaceuticals y Lundbeck. TN declara haber recibido honorarios por consultoría, ponencias y becas de viaje de Eli Lilly, Glenmark, Lundbeck, Novartis, Pfizer, St. Jude Medical, Teva Pharmaceuticals y UCB; y haber participado en comités asesores o como investigador principal en ensayos clínicos para AbbVie, Amgen, Eli Lilly, Lundbeck, Neurocrine, Novartis, Teva Pharmaceuticals y UCB. ACP declara haber impartido conferencias, participado en comités asesores o haber sido investigador principal en ensayos clínicos para Allergan, AbbVie, Pfizer, Teva Pharmaceuticals, Novartis, Lundbeck, Eli Lilly y Roche. IPM declara haber impartido conferencias, participado en comités asesores o haber sido investigador principal en ensayos clínicos para Allergan-AbbVie, Teva Pharmaceuticals, Novartis, Lundbeck y Eli Lilly. MLS declara haber impartido conferencias, participado en comités asesores o haber sido investigador principal en ensayos clínicos para Pfizer, Teva Pharmaceuticals, Novartis, Lundbeck, AbbVie y Eli Lilly. CT declara honorarios personales por su participación en consejos asesores o simposios para AbbVie, Dompé, Eli Lilly, Lundbeck, Novartis, Pfizer y Teva Pharmaceuticals; declara honorarios institucionales por la realización de ensayos clínicos para AbbVie, Dompé, Eli Lilly, Lundbeck, Novartis, Pfizer y Teva Pharmaceuticals; recibió financiación para proyectos de investigación de la Comisión Europea, el Ministerio de Salud italiano, la Fundación para la Investigación de la Migraña y la Fundación Italiana de Esclerosis Múltiple.
-A Dutch cost-effectiveness analysis of fremanezumab versus best supportive care in patients with chronic migraine and inadequate response to prior preventive therapy. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-024-03697-x
-Este trabajo contó con el apoyo de Teva Pharmaceuticals. SW es empleado de Asc Academics y consultor de la Fundación MH Pronk Health Care Consultancy.
-JAC ha recibido remuneración por su participación en consejos asesores y por impartir conferencias para Teva Pharmaceuticals, Lilly, Novartis, Lundbeck y Pfizer. MP es director general de la Fundación MH Pronk Health Care Consultancy y consultor externo de Teva Netherlands B.V. KWMZ es empleado de Teva Netherlands B.V. MTD es empleado de Teva Pharmaceuticals. LL es exempleado de Teva Pharmaceuticals. MJP es consultor de Asc Academics.
-Guidelines of the International Headache Society for Real-World Evidence studies in migraine and cluster headache. https://journals.sagepub.com/doi/10.1177/03331024251318016
-Los autores declararon los siguientes posibles conflictos de interés con respecto a la investigación, la autoría y/o la publicación de este artículo: P.P.R. informa en los últimos 36 meses haber recibido honorarios como consultora y ponente para: AbbVie, Amgen, Dr. Reddy's, Eli Lilly, Lundbeck, Medscape, Novartis, Organon, Pfizer y Teva. Su grupo de investigación ha recibido subvenciones de investigación de Novartis, Teva, AbbVie, EraNET Neuron, RIS3CAT FEDER, AGAUR e ISCIII; y financiación para ensayos clínicos de Amgen, Biohaven, Eli Lilly, Lundbeck, Novartis, Pfizer y Teva. Es Secretaria Honoraria de la Sociedad Internacional de Cefaleas. Es miembro del Comité de Guías de Ensayos Clínicos de la Sociedad Internacional de Cefaleas. Es editora asociada de Cephalalgia, Headache, The Journal of Headache and Pain, Neurologia y Revista de Neurologia. Es la fundadora de www.midolordecabeza.org. P.P.R. no posee acciones de ninguna compañía farmacéutica. N.v.V. no tiene conflictos de interés. E.C. ha recibido honorarios de Novartis, Chiesi, Lundbeck y MedScape; su salario ha sido financiado parcialmente por la beca Rio Hortega Acción Estratégica en Salud 2017–2020 y Juan Rodés 2024–2028, del Instituto de Salud Carlos III (CM20/00217). Es editor junior de Cephalalgia. G.V. no tiene conflictos de interés. M.T.F. ha recibido honorarios de AbbVie, Novartis, Chiesi y Teva. B.W.H.v.d.A. P.J.G. informa haber recibido apoyo independiente del Consejo Neerlandés de Investigación (849200007) y de la Fundación Neerlandesa del Cerebro (HA2017.01.05). P.J.G. también informa haber recibido, durante los últimos 36 meses, una subvención de Celgene; honorarios personales de Aeon Biopharma, Abbvie, Amgen, CoolTech LLC, Dr. Reddy's, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, Shiratronics, Teva Pharmaceuticals y Tremeau; honorarios personales por asesoramiento a través de Gerson Lehrman Group, Guidepoint, SAI Med Partners y Vector Metric; honorarios por materiales educativos de CME Outfitters; regalías o honorarios por publicaciones de la Massachusetts Medical Society, Oxford University Press, UpToDate y Wolters Kluwer; y una patente para estimulación magnética para el dolor de cabeza (n.º WO2016090333 A1) cedida a eNeura sin costo alguno. M.A. declara haber recibido honorarios de AbbVie, Amgen, Eli Lilly, GlaxoSmithKline, Lundbeck, Novartis, Pfizer y Teva Pharmaceuticals, fuera del ámbito del trabajo presentado. M.A. también es editor asociado de Brain, Cephalalgia y The Journal of Headache and Pain. S.J.W. ha formado parte de los consejos asesores de Allergan y Eli Lilly Taiwán. Ha recibido honorarios por ponencias de las filiales taiwanesas de Pfizer, Eli Lilly y GSK, así como subvenciones para investigación del Consejo Nacional de Ciencia y Tecnología de Taiwán, el Hospital General de Veteranos de Taipéi y la Sociedad Taiwanesa de Cefaleas. H.C.D. es miembro del Comité de Guías Clínicas de la Sociedad Alemana de Neurología y del Comité de Ensayos Clínicos de la IHS. C.T. ha recibido honorarios por su participación en consejos asesores y/o simposios científicos de AbbVie, Dompé, Eli Lilly, Medscape, Novartis, Lundbeck y Teva. Su institución ha recibido pagos de Abbvie, Eli Lilly, Novartis, Lundbeck y Teva por ensayos clínicos patrocinados o iniciados por investigadores. G.M.T. declara haber recibido apoyo de consultoría o de la industria por parte de Abbvie, Lilly, Lundbeck, Novartis, Pfizer, Teva e Interactive Studios BV, así como apoyo independiente de la Comunidad Europea, la Fundación Holandesa del Corazón y el Cerebro, el Consejo Holandés de Investigación y Dioraphte.
-Calcitonin Gene-Related Peptide Monoclonal Antibodies: Key Lessons from Real-World Evidence.
https://www.mdpi.com/2076-3425/14/9/948
-Piero Barbanti ha recibido remuneración por servicios de consultoría, participación en un consejo asesor científico, ponencias, apoyo a la investigación y participación en ensayos clínicos u otras actividades con empresas como Abbvie, Alder, Allergan, Amgen, Angelini, Assosalute, Bayer, Biohaven, Eli Lilly, Fondazione Ricerca e Salute, GSK, Lundbeck, New Penta, Noema Pharma, Novartis, Pfizer, Organon, Teva, Visufarma y Zambon. Además, es presidente de la Asociación Italiana de Personas con Cefaleas. Cinzia Aurilia ha recibido becas de viaje de Eli Lilly, FB-Health, Lusofarmaco y Teva, así como honorarios de Novartis, Eli Lilly y Teva. Gabriella Egeo ha recibido becas de viaje y honorarios de Eli Lilly, Novartis, New Penta y Ecupharma. Bianca Orlando y Giulia Fiorentini no tienen nada que declarar.
iTeva en España. Un gigante farmacéutico israelí cuyas ganancias financian en genocidio en Palestina. https://www.nogracias.org/2025/01/01/teva-en-espana-un-gigante-farmaceutico-cuyas-ganancias-danan-la-salud-de-la-poblacion-de-palestina-profesionales-de-la-sanidad-por-palestina-espana-healthworkers4palestinespain/
iiThe heroes of Teva. https://www.jpost.com/special-content/the-heroes-of-teva-779380
iiiBoicot (acción directa) a Teva. Dirigida contra sus 3 "superventas". 1/ omeprazol, 2/ atorvastatina, 3/ pantoprazol. https://hw4p.es/blog/boicot-accion-directa-a-teva-dirigida-contra-sus-3-superventas-1-omeprazol-2-atorvastatina-3-pantoprazol
ivFrémanezumab (Ajovy°) et prévention des crises de migraine. Rev Prescrire 2020 ; 40 (444): 731-732. https://www.prescrire.org/avis-sur-les-medicaments/444-fremanezumab-ajovy-et-prevention-des-crises-de-migraine
vCGRP Monoclonal Antibodies (MABs): Risks and Side Effects. https://migrainecanada.org/cgrp-monoclonal-antibodies-mabs-risks-and-side-effects/
viDisproportionality analysis of Raynaud’s phenomenon associated with calcitonin gene-related peptide inhibitors using the Food and Drug Administration adverse event reporting system. https://www.nature.com/articles/s41598-025-87421-w
viiCGRP inhibitors. https://www.drugs.com/drug-class/cgrp-inhibitors.html
viiiCalcitonin Gene-Related Peptide Receptor. https://www.ncbi.nlm.nih.gov/sites/books/NBK560648/
ixFicha Técnica Ajovy 225 mg solución inyectable en jeringa precargada. https://cima.aemps.es/cima/dochtml/ft/1191358001/FT_1191358001.html
xAntimigraineux anti-CGRP : mi-2024, un accès au prix fort en ville. https://www.prescrire.org/strategies-de-soins/487-antimigraineux-anti-cgrp-mi-2024-un-acces-au-prix-fort-en-ville
xiAJOVY (frémanezumab) - Migraine. https://www.has-sante.fr/jcms/p_3601431/fr/ajovy-fremanezumab-migraine
xiiComparación de la eficacia de eptinezumab, galcanezumab, fremanezumab y erenumab en migraña crónica https://www.ilaphar.org/wp-content/uploads/2024/01/Org_tto_migrana_2311091.pdf
xiiiA Comparison of Fremanezumab and Eptinezumab in the Prevention of Chronic Episodic Migraine. https://wjbphs.com/content/comparison-fremanezumab-and-eptinezumab-prevention-chronic-episodic-migraine
xivFremanezumab for preventing migraine NICE
https://www.nice.org.uk/guidance/TA764/chapter/1-Recommendations
xvInforme de Posicionamiento Terapéutico de fremanezumab (Ajovy®) en la profilaxis de migraña https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT_11-2020-fremanezumab-Ajovy.pdf
xviQué queda y qué hay de nuevo en el manejo farmacológico de la migraña.
https://www.navarra.es/NR/rdonlyres/9B1E99CB-9C9E-4939-B978-856C45827EAE/491139/Bit_v29n1Mod.pdf
xviiTeva Third quarter 2025 Teva Pharmaceutical Industries Ltd. November 5, 2025 https://s24.q4cdn.com/720828402/files/doc_financials/2025/q3/Q325-Earnings-Presentation.pdf
xviiiPatricia Pozo-Rosich. Bio. http://pozorosich.com/bio
xixVall d’Hebron. Patricia Pozo Rosich. https://www.vallhebron.com/en/professionals/patricia-pozo-rosich
xxHospital QuirónSalud Barcelona. Patricia Pozo Rosich. https://www.quironsalud.com/hospital-barcelona/es/cuadro-medico/patricia-pozo-rosich
xxiPatricia Pozo Rosich. https://www.fundacionlilly.com/conocenos/organos-de-gobierno/patriciapozorosich
xxiiTeva en España. Un gigante farmacéutico cuyas ganancias dañan la salud de la población de Palestina. https://www.nogracias.org/2025/01/01/teva-en-espana-un-gigante-farmaceutico-cuyas-ganancias-danan-la-salud-de-la-poblacion-de-palestina-profesionales-de-la-sanidad-por-palestina-espana-healthworkers4palestinespain/
xxiiiGuidelines of the International Headache Society for Real-World Evidence studies in migraine and cluster headache. https://journals.sagepub.com/doi/10.1177/03331024251318016
xxivLlega a España un nuevo medicamento preventivo contra la migraña: un comprimido al día. https://www.elconfidencial.com/bienestar/2024-05-09/llega-a-espana-atogepant_3880524/
xxvLa española que más sabe de migrañas te explica su origen, las nuevas terapias y los mitos más extendidos. https://www.elconfidencial.com/bienestar/2024-05-20/entrevista-a-patricia-pozo-rosich_3885270/
xxviIndustrias sanitarias, profesionales y pacientes. Cómo evitar una relación con frecuencia tóxica (corrupta). https://accesojustomedicamento.org/industrias-sanitarias-profesionales-y-pacientes-como-evitar-una-relacion-con-frecuencia-toxica-corrupta/

Israeli rape culture. Cultura israelí de la violación sexual. ENG ESP
Zionism is inherently imbued with rape culture. El sionismo como cultura de la violación
Publicado ayer.
Vídeo. "Genocidio en Palestina. Un espejo dramático que impacta en la sanidad española" Vídeo 90 min
Encuentro en Zaragoza, preparación para el Tribunal de los Pueblos sobre la Complicidad con el Genocidio palestino en el Estado español TPCGP-25.
Publicado hace 3 días.
Gaza’s Orphan Crisis: 40,000. En Gaza, 40.000 huérfanos ENG ESP
40,000 children after losing one or both parents. 40.000 niños en Gaza sin uno o dos padres
Publicado hace 8 días.
Teva y el ejército de Israel (IDF). Teva and the Israel Defense Forces (IDF). ESP ENG
Teva apoya directamente el genocidio palestino. Teva directly supports the genocide against Palestinians.
Publicado hace 14 días.
ICE operations (USA) versus Israel occupation of Gaza. EEUU, policía de inmigración versus Israel ocupación de Gaza. ENG ESP
“Imperial-colonial boomerang” in action. El «bumerán imperial-colonial» en acción
Publicado el 13 de febrero.
Teva, growing global backlash. Teva, boicot creciente ENG ESP
Teva: politics and business over compassion and ethics. Teva: política y negocio sobre compasión y ética
Publicado el 10 de febrero.
"Si Francesca Albanese es terrorista, yo también". "If Francesca Albanese is a terrorist, then so am I" ESP ENG
"Una relatora de derechos humanos de la ONU, tratadа como terrorista por documentar el genocidio en Gaza". "A UN human rights rapporteur, treated as a terrorist for documenting the genocide in Gaza"
Publicado el 9 de febrero.
Vídeo, 60 sg. Dr. Hussam Abu Safiya. Lazos Rojos por la liberación de rehenes palestinos.
Campaña por la liberación de rehenes palestinos
Publicado el 7 de febrero.Ver más / See more


